Information about clinical trials
Information about clinical trials
At the Netherlands Cancer Institute, we treat patients with cancer from all over the Netherlands and every year, several thousand patients participate in research into new treatment methods at the Netherlands Cancer Institute. This research is called 'clinical scientific research', 'experimental research', 'clinical trial' or 'clinical trial'. We use the terms 'clinical trial' and 'trial'. On these pages you will find general information. You can discuss any options available to you with your doctor.
Our research
In addition to being a hospital, the Netherlands Cancer Institute (NKI) is also a research institute. Some 700 scientific researchers are working there to improve cancer treatments. They develop and test new drugs, look at the effect on tumors, find out what best dosages are and pay attention to possible side effects. Our researchers are also constantly working on improving diagnostics, refining surgical techniques or new ways of radiotherapy. Through all these efforts, we are getting more and more grip on cancer. Your attending physician - here or in another hospital - may point out a possibility to participate. Or you can raise this yourself. It is impossible to say in advance exactly what participating in a clinical trial can mean for you. The possible effect on your disease depends on many factors. Your doctor will discuss this with you.
-
450
-
2500

Part of our ongoing studies
Different phases of clinical research
A new treatment goes through several phases. Each phase has a different goal.
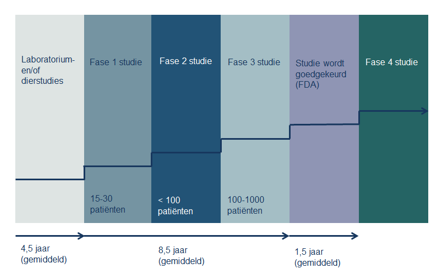

Phase 1 study
Phase 1 study:
A phase 1 study looks at how the human body reacts to the drug. Research into new medicines often also examines how the drug behaves in the body, how it is broken down and which form of administration is most suitable (tablet or infusion). At this stage, it is not yet a question of whether the drug has an effect on the tumor. For example, researchers want to know the best way to administer it and see how the drug behaves in the body.
If the study concerns treatment with a new drug, the chance that you will benefit from this treatment yourself is usually small. A phase 1 trial may also focus on a new administration schedule or a new combination of treatments. In this case, there is already data about the effect and side effects of the treatment and there is the possibility that a patient will benefit from the examination.
Read more about Phase 1 studies here.
Read more about the Center for Early Clinical Research here.
Phase 2 study
A phase 2 study tests whether a certain type of cancer responds to a new treatment. So does the drug have the desired effect on the tumors: are the cells really destroyed or growth inhibited?
Phase 3 study
In phase 3 studies, the effect of the new treatment with regular drugs is compared. If the new drug turns out to work well and safely, it will be registered and put on the market.
Finally, there will be a lengthy fourth phase in which the long-term effects of the drug will be studied, what are the long-term benefits and side effects?
Participation in the survey
Your doctor will inform you about the study and give you written information about it. Please take the time to go through this information and possibly discuss it with others.
In most cases, a separate information meeting with a specialized nurse is also arranged. The pros and cons, how the study works, consequences, side effects, and additional burden that the study may have on you will all be discussed with you. Participation in the study is of course entirely voluntary. If you decide to participate, you will be asked to sign. Your formal consent is called 'Informed Consent'. This is kept in your medical file. You can withdraw your consent at any time of your choosing.
Important to know
1. Participation in a clinical trial does not limit your access to other care
If a clinical trial is unsuccessful in treating your cancer, you may consult with your doctor to stop your participation and start another treatment. In some cases, you can enrol in another clinical research study.
2. Clinical research is safe
Patient safety is the main concern at the Netherlands Cancer Institute. The research is designed by experts and goes through several approval rounds. Before patients can participate, the protocol is reviewed and approved by the Medical Ethics Committee, a committee consisting of doctors, nurses, researchers, patients, and advocates. Only after these and other approvals can the research begin. There is also a law in the Netherlands that states the conditions under which scientific research may take place in humans, the 'Medical Research Involving Human Subjects Act (WMO)'. The Medical Ethics Committee assesses, among other things, whether the research protocol meets the conditions of the WMO and whether the interests of the patient are sufficiently protected.
3. Clinical research is for patients in all stages of cancer
While all studies have criteria for participants, clinical trials are available for patients at all stages of cancer. The criteria of a study, which are intended to ensure patient safety, may include the patient's age, gender, cancer type and stage, previous treatments, and overall health. Your doctor is the best resource for knowing your treatment options.
4. In some studies, a lottery takes place
To see whether a certain treatment is effective and useful, a study is sometimes carried out 'randomly'. This means that testing is done in an intervention group and that the results are compared with a control group (the group of people who are not treated with the drug).
5. You can also participate in an NKI study if you are being treated in another hospital
It does mean that we take over your treatment and you must therefore come to the NKI regularly.
6. Participation in a clinical trial is usually reimbursed
The sponsor of a study usually reimburses the costs of participating in clinical trials. In the event that regular care also takes place in addition to the study, this is usually reimbursed by the health insurer.

AVL Foundation
More research Less cancer
 nl
nl


