Information about clinical trials
Information about clinical trials
At the Netherlands Cancer Institute, we treat patients from all over the country. Every year, several thousands receive their treatment as part of our research into new therapies. This kind of research is called “clinical science research”, or “experimental studies”. We prefer to use the term “clinical trials” on our websites. Please find general information about our trials on this page. To find out whether there is a trial available for you, please consult your physician.
Our research
The Netherlands Cancer Institute is more than just a hospital: approximately 700 scientific researchers at our institute are working towards improvements in cancer treatment. These scientists develop and test new drugs, monitor their effects on tumors, try to find the proper doses, and keep a close eye on the potential side effects. Our researchers are permanently involved in the development of improvements in diagnostics, refinements of our surgery techniques, and new ways to deliver radiation. Because of their efforts, we can increase our grip on cancer. Your practicing physician – at the NKI or at another hospital – may ask you to consider participating in one of our trials, or you may want to ask your physician about your options. We cannot predict what your experience with the clinical trial will be like. The treatment’s effects on your illness will depend on various factors. Your physician can discuss your personal situation with you.
-
450
Within the Nederlands Cancer Institute there's an average of about 450 clinical studies. -
2500
Every year about 2500 to 3000 patients of the NKI participate in a study. That is more than 20 percent.

The different phases of clinical research
New treatments have to go through several trial phases before they can be used in the clinic. Every phase has its own goal.
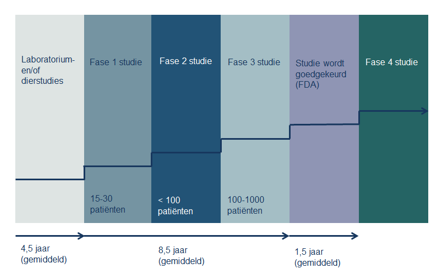

Phase 1 trial
Phase 1 trial:
A phase 1 trial looks into the ways in which the human body responds to the drug. When researching new drugs, we often want to know how the substance behaves in the body, how it is metabolized, and how it can best be administered into the body (pill or IV). We aren’t concerned with the effects that the substance may have on the tumor at this phase. Researchers want to make sure that they know how to administer it, and how it will behave in the body. If the trial involves treatment with a new drug, you may not experience any benefits of your participation yourself. A phase 1 trial may also involve a new drug schedule or a new combination of treatments. That means that we do know a little bit about the way the drug(s) work, and their potential side effects, so the treatment you receive may benefit you.
Click here to learn more about Phase 1 trials.
Click here to learn more about our Clinical Pharmacology Department.
Phase 2 trial
A phase 2 trial looks into the way certain types of cancer respond to a new treatment type. Does the drug have the desired effect on the tumor? Does it destroy the tumor cells or halt their growth?
Phase 3 trial
A phase 3 trial compares the results of the new treatment to the standard treatment. If the new treatment works well and is safe, it will be registered and made available.
What follows is a very long phase 4 trial which monitors the effects of the drug over a longer period of time: what are its long-term advantages and side-effects?
Your participation
Your physician will inform you about the trial, and provide you with booklets or flyers with more information. Please read them carefully, and feel free discuss them with others.
Most people will be invited to attend an additional informative consultation with a specialized nurse. He or she will discuss the pros and cons of participation, what you can expect during the trial, the consequences, side-effects, and the additional patient burden. Your participation is completely voluntary. If you do decide to participate in the trial, you will be asked to sign a form. We call this your “informed consent”, and will keep it in your medical file. You are free to withdraw your consent at any time.
Important information
1. Your participation in a clinical trial will not restrict your access to other care
If the clinical trial was unsuccessful in treating your cancer, you and your physician can decide to stop your participation in favor of a different kind of treatment, or consider participating in a different experimental study.
2. Clinical science research is safe
Patient safety is the Netherlands Cancer Institute’s primary concern. Our trials are designed by experts and will have to pass several rounds of inspection before opening up for patients. The Medical Review & Ethics Committee has to evaluate and approve of this protocol. This committee consists of physicians, nurses, researchers, patients, and lawyers. Only after review and approval can we start our trials. The Dutch Medical Research Involving Human Subjects Act (Wet medisch-wetenschappelijk onderzoek met mensen; WMO) states under which precautions scientific research involving people can be conducted. The Medical Review & Ethics Committee also checks whether the study is in accordance with the WMO, and whether the rights of the patients are sufficiently protected.
3. Clinical science is available to patients with cancer of any stage
Although every trial has its own inclusion criteria, trial participation is open to all patients, no matter the stage of their disease. Some possible inclusion criteria are: age, gender, cancer type or stage, previous treatment(s), and general health and wellbeing. Your physician can tell you more about your options.
4. Some trials are randomized
To ensure that a treatment is effective, some trials are randomized. This means that we treat an intervention group with the new treatment and compare their results with a control group (people who do not receive the new drug).
5. You are welcome to participate in a trial at the NKI, even if you are receiving treatment at a different hospital
In order to participate, you will have to transfer to our hospital for treatment, which means that you will have to come to the NKI regularly.
6. Participation in a study is usually reimbursed
The sponsor of a study usually reimburses the costs of participating in clinical trials. In the event that regular care also takes place alongside the study, this is usually reimbursed by the health insurance company.

 nl
nl
 Nederlands
Nederlands
