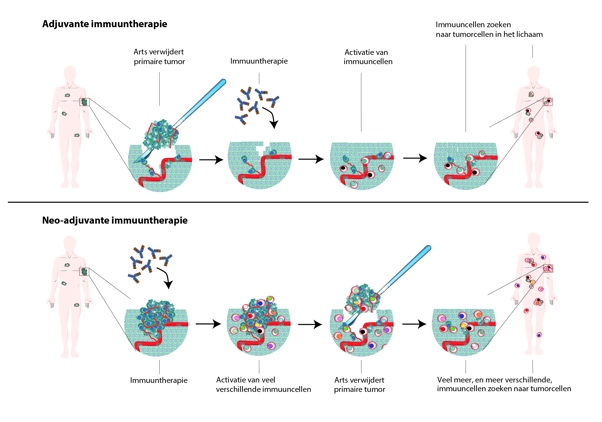
The study investigated nearly 100 patients receiving immunotherapy for stage 3 melanoma before surgery. After the therapy, the immune system had to start attacking tumor cells. A rapid response appears to have an important predictive value, the study showed.
98% of the 71 patients who displayed a rapid response did not relapse within two to four years. Out of the small group of patients who did not show an immune response, two out of three saw a recurrence of the cancer. Blank is working on new treatments for these patients to improve their chances as well.
In the second publication, researchers compared different neoadjuvant therapies (therapies that precede surgery) in patients with melanomas, focusing on the relationship between patients’ response rates and their survival rates without recurrence. The research combined data from six different studies.
The researchers compared four different therapies: two immunotherapies and two targeted therapies. Targeted therapy consists of drugs that inhibit tumor cell division.
The most important result: if patients responded well to the neoadjuvant treatment (pathological response), there was almost no recurrence. This was most prominent in immunotherapy compared to targeted therapy. A plausible explanation is that immunotherapy activates the immune cells to attack the tumor cells, which continues to occur once the therapy is completed.
That could explain why even a partial pathological response to immunotherapy proved to have a highly beneficial long-term effect. Without this response, the prognosis for all therapies was significantly worse. Blank: “This is important news for drug administration agencies like the FDA or EMA: early immune response is an important indicator for the efficacy of the therapy.”
 nl
nl
 Nederlands
Nederlands